views
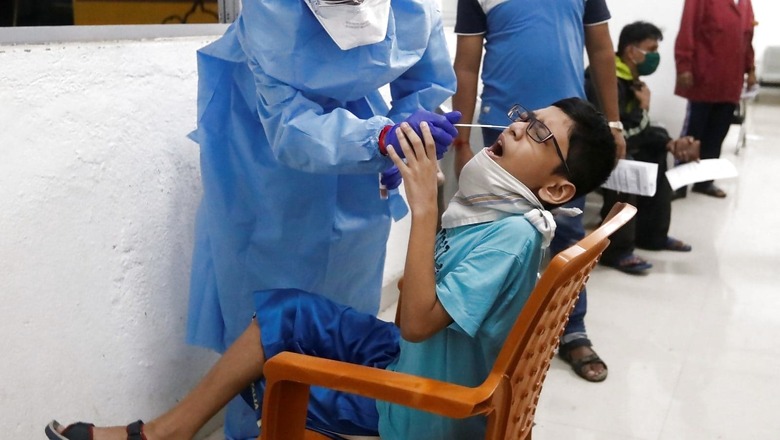
Covid-19 vaccines should be made available by September for children, AIIMS Director Dr Randeep Guleria has said. Seeking to quell concerns around reopening of schools, and rising cases of paedriatic Covid-19 cases, Guleria said the jabs should be made available for children soon and thus the government can start considering the opening schools in a graded manner in areas where the positivity rate is less than 5 percent.
Even as experts say that the children rarely develop severe forms of Covid-19, the states of Karnataka and Maharashtra have recently seen a spike in paediatric Covid cases, and some deaths too. Not only instances of coronavirus, some cases of paediatric black fungus have been reported too.
World Health Organization’s chief scientist Soumya Swaminathan had told News18 that till vaccines are available for children, more and more parents and teachers need to get vaccinated to protect them from contracting the virus. “I am very hopeful that ultimately we’ll have vaccine for children. But that’s not going to happen this year, and we should open schools when community transmission is down. That’s what rest of the countries have done, with other precautions. And if teachers are vaccinated, that would be a big step forward,” WHO’s top scientist added.
Children aged 12-15 are already being jabbed in the US, Canada and the EU, while the United Kingdom has also approved the Pfizer/BioNTech vaccine for that age group.
According to a report in The Guardian, the Moderna jab, which is made in a similar way, is also approved for children in the US. They can both be used for older teenagers, since the main trials involved young people over the age of 16. AstraZeneca is now trialling its vaccine in younger children between the ages of six and 17. It is currently approved only for over-18s.
Status of Covid Vaccines for Children in India
Covaxin: India’s drug regulator – Drugs Controller General of India (DCGI) – to Bharat Biotech for conducting trials of its vaccine on 2-18-year-olds. Recently, a petition was filed in the Delhi high court for setting aside the permission granted for II/III clinical trials on children.
Dr Paul, however, said that both Covaxin and Zydus Cadila’s vaccine are being tested on children — indicating that these two vaccines are potential vaccinates to immunise children. The vaccine is already under trial at AIIMS Patna.
Zydus Cadila vaccine: Ahmedabad-based drug company is likely to get emergency use nod for its Covid-19 vaccines for children above 12 years this week. The regulator’s subject expert committee (SEC) will examine data submitted by Cadila. If the SEC finds the phase 3 data of the vaccine company satisfactory, the emergency use authorisation for the vaccine can be granted this week itself. Officials said that if the approval is given, supply of the vaccine is expected to start by August-September.
Pfizer: AIIMS director Dr Randeep Guleria said that Centre’s decision to waive off indemnity to Pfizer will not only help adults, but children too. Talking to News18, he said, “This has been done in the past too when the government gave emergency approvals to all vaccines that had been approved by agencies of US, UK or EU and WHO. Based on that, emergency approvals have already been given de-facto to vaccines with approvals from these agencies and the issue of indemnity also seems to be resolved. So, I think we will have Pfizer vaccine coming in for children and adults shortly.”
Sputnik V: Russian news agency TASS in May quoted director of Gamaleya National Research Center for Epidemiol as saying that trials of the Sputnik V vaccine against the coronavirus infection in children may begin in the next few weeks provided a corresponding permit of the Russian Health Ministry is obtained. The developer explained that during the trials, the children, depending on their age and weight, will be administered different doses of the Sputnik V vaccine. The formulation of the preparation itself won’t be altered.
While Sputnik V doses for adults will be available in India from June-end, there have been no talks for a tie-up for paediatric vaccines.
Moderna: Moderna recently said its coronavirus vaccine is effective and it strongly protects kids as young as 12. In adolescents aged 12-17, Moderna’s Covid-19 vaccine showed no new or major safety problems in a clinical trial, the developer has said. If the vaccine is approved in the US, it will become the second option for vaccinating children aged between 12 and 17. However, there have no talks for bringing the vaccine in India for children.
Severity of Covid-19 in Children
It has come to light that on rare occasions, children who have experienced even mild infections can later develop a sometimes deadly condition called multi-system inflammatory syndrome in children (MIS-C).
Considering the recent study of paediatric cases, India is too looking at vaccinating children. Dr V K Paul, the head of country’s Covid-19 task force, has said the decision on vaccinating children is being “continuously examined” and emphasised that once the rollout for the children takes place, all of them have to be covered at the same time.
“We will have to take this into account and strategise. The first is which vaccine to give… Yes, currently vaccine ‘A’ (Pfizer) is suitable…but once you have to take a decision, we have to keep in mind who were are trying to cover….I can assure you that this is continuously being examined,” Paul was quoted as saying by Indian Express.
Often the Opposition parties in India have raised questions on the status of vaccines for children. Replying to them, Union minister Hardeep Singh Puri said, “Rahul Gandhi asks where is the vaccine for our children. That vaccine is in the garbage in Rajasthan and profits are being made on that vaccine in Punjab. This is the culture of Congress.” Apart from the political slugfest, what exactly is the situation of coronavirus vaccines for children?
Read all the Latest News, Breaking News and Coronavirus News here.




















Comments
0 comment