views
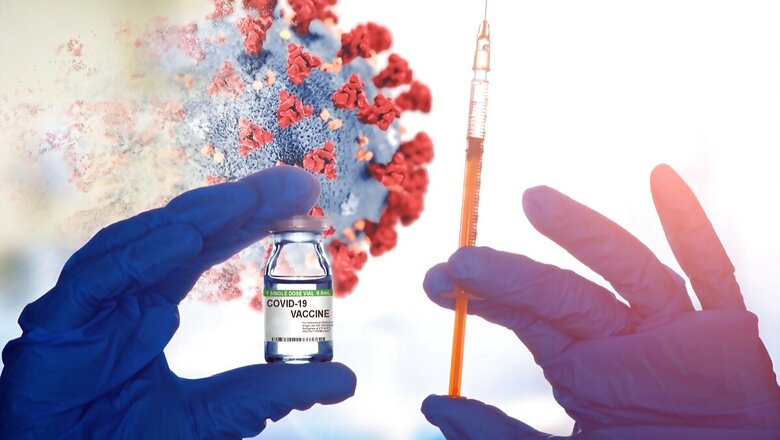
The Brihanmumbai Municipal Corporation has written to the Central government seeking permission to conduct trials of Bharat Biotech’s vaccine — Covaxin — on children of 12-18 ages. Speaking to CNN-News18, Additional Municipal Commissioner Suresh Kakani on Friday said, “We have conveyed our willingness to conduct trials in kids aged between 12-18. We are awaiting a nod from that agency, so that we can take it forward. We have to take permission of the ethics committee, once the nod is given.”
The move comes after vaccine companies wrote to BMC for the trials, he said. On the question of where the trials will be conducted, he said, “As of now, we have conveyed willingness of the two medical college hospitals and, we are not aware of the sample size, but we definitely go by the ICMR’s guidelines.”
Meanwhile, Patna’s All India Institute of Medical Sciences (AIIMS) has started the paediatric trials for Covaxin after Bharat Biotech received the nod from Drugs Controller General of India (DCGI) for clinical trials on May 11. AIIMS Delhi is also planning to start paediatric clinical trials of Covaxin in the coming day, news agency ANI reported.
Earlier, VK Paul, Member (Health), Niti Aayog had said, “Covaxin has been approved by the Drugs Controller General of India (DCGI), for Phase II/III clinical trials in the age group of 2 to 18 years.”
Several experts have warned that the third wave will likely affect children, as Singapore has already reported.
Read all the Latest News, Breaking News and Coronavirus News here.



















Comments
0 comment