views
Calculating the Dilution Formula
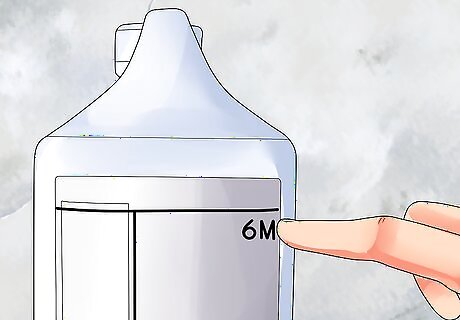
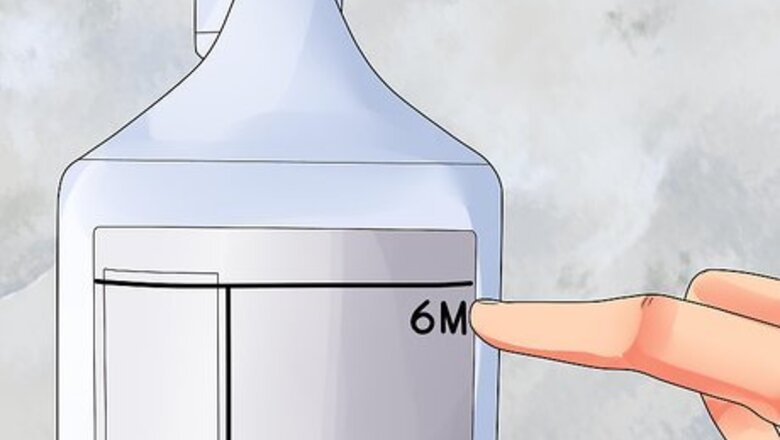
Check what you already have. Find the concentration of the acid solution on the label, or in the word problem you are working on. This number is often written in units of molarity, or molar concentration, abbreviated as M. For example, a "6M" acid contains six moles of acid molecules per liter. We'll call this starting concentration C1. The formula below will also use the term V1. This is the volume of the acid we will be adding to water. We probably won't use the entire bottle of acid, though, so we don't know what this number is yet.

Decide on an end result. The desired concentration and volume of acid is typically determined by a school assignment or by the requirements of the laboratory you are working in. For example, we might want to dilute our acid to a concentration of 2M, and need 0.5 liters (0.1 US gal). We'll call this desired concentration C2 and the desired volume V2. If you are using unusual units, convert them all to units of molar concentration (moles per liter) and liters before you continue. If you are not certain what concentration or volume of acid is required, consult your teacher, a chemist, or an expert in the task you plan to use acid in.
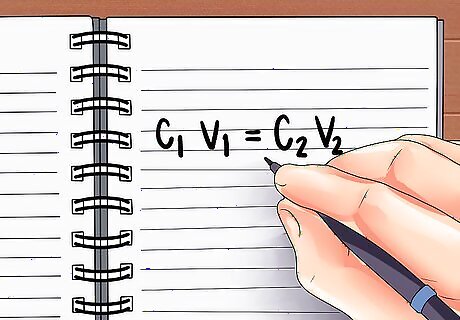
Write the formula for calculating dilution. Whenever you prepare to dilute a solution, you can use the formula C1V1 = C2V2. words, this means "the initial solution's concentration x its volume = the diluted solutions' concentration x its volume." We know this is true because concentration x volume = the total amount of acid, and the total amount of acid will remain the same as it is added to the water. In our example, we can write this formula (6M)(V1)=(2M)(0.5L).

Solve the formula for V1. This term, V1, will tell us how much of the initial acid solution we need to add to water in order to end up with the desired concentration and volume. Rearrange the formula as V1=(C2V2)/(C1), then plug in the numbers you know. In our example, we would end up with V1=((2M)(0.5L))/(6M)=⁄6 liter (0.0 US gal). This approximately equals 0.167 liters (0.0 US gal), or 167 milliliters.

Calculate how much water you'll need. Now that you now V1, the amount of acid you'll be using, and V2, the amount of solution you'll end up with, you can easily calculate how much water you need to make up the difference. V2 - V1 = the volume of water required. In our case, we want to end up 0.5 liters (0.1 US gal) and will be using 0.167 liters (0.0 US gal) of acid. The amount of water we need = 0.5L - 0.167L = 0.333L, or 333 milliliters. Always double-check your calculations to make sure that they're accurate.
Preparing a Safe Workspace

Read the relevant Chemical Safety Cards online. International Chemical Safety Cards provide succinct, detailed safety information. Search for the exact name of the acid you will be using, such as "hydrochloric acid," in the online database. Some acids may require additional safety precautions, besides those described below. Sometimes several cards are issued, depending on the concentration and additions to the acid. Pick the one that most closely matches your initial acid solution.

Wear splash goggles, gloves, and a lab coat. Splash safety goggles that cover all sides of the eyes are required when handling acid. Protect your skin and clothing by wearing gloves and a lab coat or apron. Tie up long hair before handling the acid. Acid can take several hours to burn holes through clothing. Even if you do not notice a spill, a few drops can damage your clothing if it is not underneath a lab coat.

Work in a fume hood or ventilated area. Whenever possible, keep the acid solution in a functioning fume hood while you are working. This limits exposure to gaseous vapors produced by the acid, which can be corrosive or poisonous. If a fume hood is not available, open all windows and doors, or turn on a fan to ventilate the area.

Know where running water is located. If acid gets on your eyes or skin, you'll need to flush it with cool, running water for 15–20 minutes. Don't start the dilution until you know where to find the nearest functioning eye wash station or sink. When washing your eyes, keep your eyelids wide open. Rotate your eyes by looking up, right, down, and left to make sure all sides of your eyeball are rinsed.
Have a spill plan ready, specific to your acid. You can purchase an acid spill kit that contains all the necessary materials, or acquire neutralizers and absorbers separately. The process described here can be used for hydrochloric, sulfuric, nitric, or phosphoric acid, but other acids may require further research to dispose of properly: Ventilate the area by opening windows and doors, and turning on fume hoods and fans. Apply a weak base such as sodium carbonate (soda ash), sodium bicarbonate, or calcium carbonate to the outer edges of the spill, avoiding the resulting spatter. Continue to apply slowly, working inward, until the spill is covered. Mix well with a plastic tool. Check the pH of the spill with litmus paper. Add more base if necessary to get the pH between 6 and 8, then flush the spill down a drain with plenty of water.
Diluting the Acid
Cool water in an ice bath when using concentrated acids. This step is only necessary when you'll be handling extremely concentrated acid solutions, such as 18M sulfuric acid, or 12M hydrochloric acid. Cool the water you'll be using by keeping it in a container surrounded by ice for at least 20 minutes before the dilution begins. For most dilutions, the water can be at room temperature.
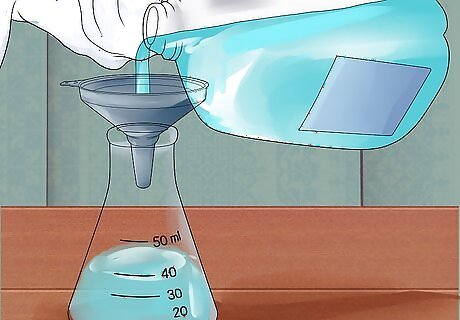
Add distilled water to a large flask. For projects involving careful measurement, such as titration, use a volumetric flask. For most practical purposes, an Erlenmeyer flask can be used instead. In either case, choose a container that can easily contain your total desired volume, with plenty of space remaining, to minimize splashes over the rim. You don't need to carefully measure this water, as long as it came from a container that was carefully measured to contain the total required amount of water.
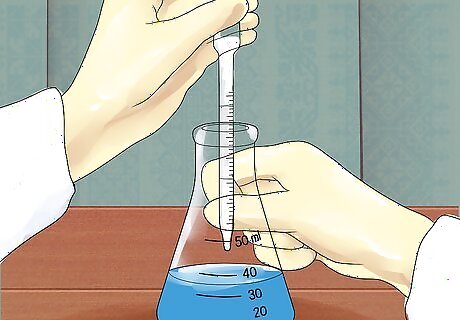
Add a tiny quantity of acid. If using a small volume of acid, use a graduated (Mohr) pipette or volumetric pipette with a rubber bulb on top. For larger volumes, place a funnel in the neck of the flask, and slowly pour in a small quantity of the acid using a graduated cylinder. Never use a mouth pipette in a chemistry lab.

Allow the solution to cool off. Strong acids may generate lots of heat when added to water. If the acid was highly concentrated, the solution may splatter or produce corrosive fumes. If this happens, you will need to perform the entire dilution in very small doses, or cool the water in an ice bath before you continue.
Add the remaining acid in small doses. Allow the solution time to cool off between each dose, especially if you notice heat, fumes, or spatter. Continue until the required amount of acid has been added. This amount was calculated as V1 above.
Stir the solution. For best results, you can stir the solution with a glass stirring rod after each addition of acid. If the size of the flask makes this impractical, stir the solution after the dilution is complete and the funnel is removed.
Store the acid and rinse the tools. Pour the acid solution you created into a clearly labeled container, preferably a PVC coated glass bottle, and store in a safe location. Rinse the flask, funnel, stirring rod, pipette, and/or graduated cylinder in water to remove all traces of acid.



















Comments
0 comment