views
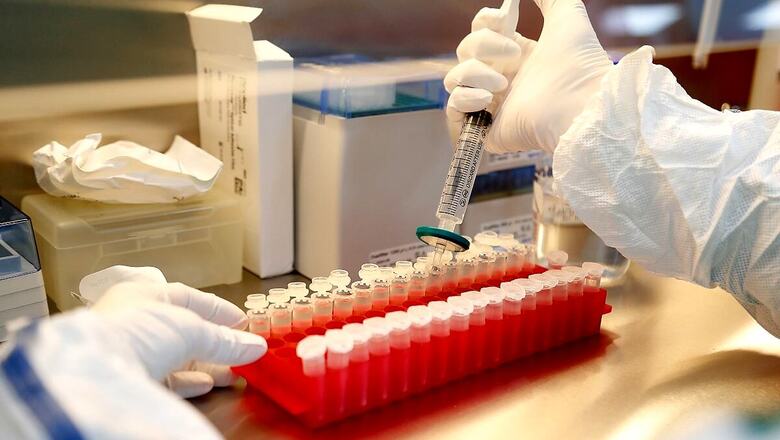
The phase-3 clinical trials of Bharat Biotech's COVID-19 vaccine began at Sola civil hospital in Gujarat's Ahmedabad city on Thursday, officials of the state-run hospital said. On the first day, five healthy volunteers, including a woman, were given the first dose of the vaccine at the hospital. Its second dose will be given after 28 days, said Principal Investigator, Sola Civil, Dr Parul Bhatt.
"In the first phase, we have planned to cover 1,000 healthy volunteers. They must be in the age group of 18 to 60 and with no history of coronavirus infection. Anyone who fulfils the criteria can approach us and enrol as a volunteer," she added. Covaxin is being developed by the Hyderabad-based firm Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR).
She said that doctors at the hospital engaged in the clinical trial exercise will remain in touch with the volunteers over phone to monitor their health condition after the vaccine is administered. Volunteers will be called at the hospital at regular intervals for follow-up procedures and tests, including blood test and oxygen level, Bhatt said.
"This whole exercise will continue for 12 months. We will also give a contact number to the volunteers in case they need any urgent intervention. The hospital will provide necessary treatment in case of any complication," she added.
Read all the Latest News, Breaking News and Coronavirus News here




















Comments
0 comment