views
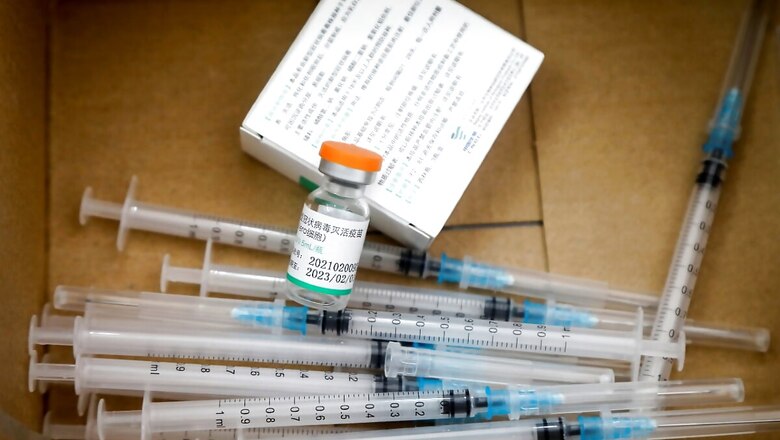
The government is looking to ramp up the vaccine production and distribution across the country as second wave of coronavirus continues to plunge India into healthcare crisis. While the Centre revised rules to expedite foreign vaccine acquirement, it is also stepping up vaccine production in the country, Moneycontrol reported. Shortage of jabs and sluggish vaccination drive has only added to the whammy of coronavirus spike in India.
Support is being extended at multiple levels to domestic vaccine manufacturers, Moneycontrol quoted sources as saying.
The government is planning to augment facilities at private and public vaccine manufacturing units like Bharat Biotech as well as Indian Immunologicals in Hyderabad, Haffekine Biopharmaceuticals in Mumbai and Bharat Immunologicals and Biologicals in Bulandshar, according to report.
The capacities of Bharat Biotech Limited, Hyderabad as well as other public sector manufacturers are being upgraded with the required infrastructure and technology. Financial support is being provided as a grant from Centre to the tune of appx Rs 65 crore to Bharat Biotech’s new Bangalore facility which is being repurposed to increase the capacity of vaccine production, the statement said.
Production of indigenously developed Covaxin vaccine of Bharat Biotech will be doubled by May-June and then increased nearly 6-7 fold by July – August 2021,implying, from one crore vaccine doses, Bharat Biotech will produce 6-7 crore doses per month in July and August, with a financial aid of Rs 200 crore.
Apart from Bharat Biotech, the Centre is planning to augment facilities at other private and public vaccine manufacturing units like Indian Immunologicals in Hyderabad, Haffekine Biopharmaceuticals in Mumbai and Bharat Immunologicals and Biologicals in Bulandshar, according to reports.
The ramp up in production along with Centre’s recent move of fast track approval to foreign-made vaccines is being done to overcome shortfalls in vaccination drive, which is being seen by experts as one of the few options available to tackle the virus spread. The government is also reportedly providing advance payments for supplies to domestic vaccine manufacturers.
Further to augment the vaccine production process, an inter-ministerial group has been constituted. The group has is in talks with vaccine manufacturers to upscale the domestic manufacturing capacities. Financial and institutional support is also being extended to the indigenous manufacturers. According to reports, Indian Council of Medical Research (ICMR) provided Rs.10 crore assistance to SII for conducting the trial of Covishield vaccine. The medical body also extended Rs.10 crores assistance to SII for Novavax trials. It also provided assistance to Bharath Biotech for Covaxin vaccine trials, Zydus Cadilla for conducting pre-clinical animal trials for their Covid vaccine.
The Department of Biotechnology (DBT) is also helping vaccine manufacturers for the development of covid vaccine candidates. DBT and its Public Sector Undertaking, Biotechnology Industry Research Assistance Council (BIRAC), are supporting nearly 15 vaccine candidates at a cost of about Rs 100 crore.
Under its Atmanirbhar Bharat 3.0, the Department of Biotechnology announced Mission Covid Suraksha programme, at a cost of Rs 900 crore for 12 months. Five vaccine candidates in the advanced clinical development stage are being supported under this Mission. Nineteen clinical trial sites across India, 03 immunogenicity assay laboratories and three animal challenge facilities are also being supported under the Mission.
Around 11 Good Clinical Laboratory Practice (GCLP) Compliant clinical trial sites have been established to ensure quick clinical trials. Each site has access to a cohort of about 50,000 – 1,00,000 healthy volunteers, along with 34 hospital sites being marked as vaccine trial sites.
The drug regulatory body, Central Drugs Standard Control Organization (CDSCO), has taken initiatives to fast track approvals of new vaccines. For instance, notifications are issued to enable stockpiling of the vaccine while it is under clinical trial.
Additionally, on Thursday, the government issued a regulatory order saying that India’s drug regulator will take decision on applications seeking approval for restricted emergency use of foreign produced vaccines within three working days from date of submission. The move comes at a time when India is faced with an alarming surge in coronavirus cases.
Earlier this week, Russian vaccine Sputnik V received a nod from India’s expert panel for emergency use. Dr Reddy’s Laboratories will start importing bulk doses of its anti-coronavirus vaccine Sputnik V from Russia between mid-April to June. According to a contract signed between Hyderabad-based Dr Reddy’s Laboratories (DRL) and Russian Direct Investment Fund (RDIF), India will receive about 250 million doses of Sputnik V that would be sufficient for 125 million people. Other vaccines in the pipeline for emergency use in India are Johnson &Johnson, Pfizer, Moderna, Zydus Cadila and Novavax.
Read all the Latest News, Breaking News and Coronavirus News here. Follow us on Facebook, Twitter and Telegram.



















Comments
0 comment