views
The government will soon deploy a Made-in-India finger prick diagnostic test to detect sickle cell anaemia under the National Sickle Cell Anaemia Elimination Mission.
With the goal of eradicating sickle cell anaemia from India by 2047, the Indian Council of Medical Research (ICMR) has approved the first “Made-in-India” sickle cell rapid testing kit.
The mission aims to cover seven crore people for screening to identify people with sickle cell disease in the next three-and-a-half years up to the financial year 2025-26.
Sickle cell disease (SCD) is a genetic blood disorder where red blood cells contort into a sickle shape. In this case, the human body faces trouble in making enough healthy red blood cells and undergoes several blood transfusions, increasing the chances of chronic infections.
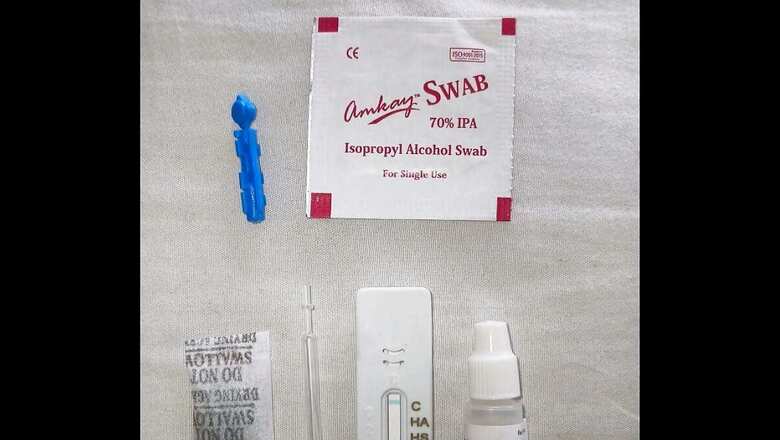
A finger-pricking test kit — manufactured by Maharashtra-based Voxtur Bio — will be procured by the central and state governments for screening of tribal and other at-risk populations under the programme.
Similar to the pregnancy testing kit, its ease of use and instant results (within 10 minutes) could help India achieve its targets faster. The device — unlike other available tests where samples are taken and sent to laboratories for evaluation — could become a game-changer in India’s fight against the genetic blood disorder.
“The rapid testing kit saves time, eliminates the need for skilled manpower and additional resources which are required to draw blood, transport samples to laboratories and later, generate reports,” Dr Veeraal Gandhi, chairman and managing director, Voxtur Bio, told News18.
“With our testing kit, results are available then and there without the need for skilled technicians and doctors at laboratories to interpret the results.”
Also, the company claims to be the first to roll out Covid-19 antibody test kits or ferritin semi-quantitative test kits in India. “We have been pioneers in manufacturing rapid testing kits. However, sickle cell kit has been developed by us in the shortest possible time,” Gandhi said, adding that his firm started developing the kit in December and by March, it had already applied for a licence.
In February, during the Union Budget 2023 presentation, Finance Minister Nirmala Sitharaman had also announced the mission to eliminate sickle cell anaemia, giving a further boost to the project.
How does it work?
The kit, which is evaluated by ICMR, claims to have 100 per cent specificity, sensitivity and accuracy.
The kit is made to diagnose four types of sickle cell conditions which are more common in India — Sickle Cell Trait (HbAS), Sickle Cell Disease (HbSS), Sickle HbC Disease (HbSC) and Sickle HbCTrait (HbAC).
The kit works similar to a pregnancy testing device but requires two drops of blood (mixed with an assay) dispensed over the tray in the test card.
“Within 10 minutes, a control band would appear in front of any four traits. If it does not appear, the test would be considered invalid,” Gandhi explained.
Testing kit for thalassemia coming soon
Gandhi, who is also the vice president of the Association of Diagnostics Manufacturers of India (ADMI), told News18 that his company is working on a similar product for another genetic blood disorder — thalassemia.
While he did not disclose more information on the product and expected launch timelines, Gandhi said post-Covid-19, diagnostics had moved out of laboratories into homes.
“Now, the idea behind developing products is to find a high-tech device which is easier to reach patients. In short, the industry is now working on making testing simpler.”
In India, the sickle cell rapid testing kits will be sold to the Centre. However, the company will export the product to other markets, including Africa, Mediterranean regions, sub-Saharan regions, Central America and other destinations.




















Comments
0 comment