views
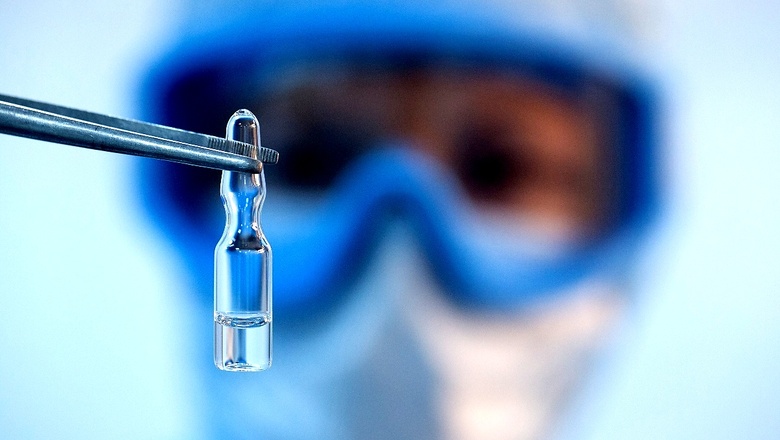
The Council of Scientific and Industrial Research (CSIR), India’s premier research organisation, and Mylan Laboratories Ltd on Wednesday announced a partnership to address unmet patient needs amidst the evolving Covid-19 pandemic.
Under the partnership, CSIR’s constituent laboratory, the Indian Institute of Chemical Technology (CSIR-IICT), and Mylan will collaborate to identify potential therapies for Covid-19.
A series of clinical trials will be conducted towards new and innovative solutions to manage the Covid-19 pandemic in India as part of this collaboration. The first of the clinical trials to be rolled out is a multiple arm Phase-3 study that will be conducted in adult patients with mild to moderate Covid-19 at risk of complications.
CSIR Director General, Dr. Shehkar C Mande said: “The current collaboration with Mylan is a significant milestone and during the current Covid-19 pandemic, CSIR has prioritised conducting clinical trials of well-proven drugs in partnership with industry towards the development of multiple therapeutic options for Covid-19.”
CSIR-IICT Director, Dr Chandrasekhar said: “CSIR is delighted to associate with Mylan as knowledge and scientific partner, and looks forward to working with the company, especially given Mylan’s vast industry experience in clinical trials and commercialisation.”
Mylan Chief Operating Officer, Sanjeev Sethi said that the collaboration with the CSIR is a strategic step forward aimed at identifying effective treatments for patients with Covid-19.
“In addition to bringing forward new indications, this partnership will also help us identify multiple molecules that can potentially be leveraged in therapies for various other infectious diseases in the future.”
The application for the clinical trials has been submitted to the Drugs Controller General of India (DCGI) for regulatory approval. CSIR has appointed Dr. Ram Vishwakarma, Honorary Advisor to the DG-CSIR and former Director CSIR-IIIM (Indian Institute of Integrative Medicine) as a mentor to lead this collaboration.



















Comments
0 comment