
views
India now has its first indigenous kit that will detect tuberculosis and other drugs resistant to first-line anti-TB, rifampicin and isoniazid.
The Pune-based biotech company, Mylab Discoversy Solutions, started working on a tuberculosis detection kit four years ago but the project got delayed by Covid-19 outbreak in 2020.
The kit has been recently approved by the country’s health regulator and the Indian Council of Medical Research (ICMR).
In 2020, Mylabs became India’s first company to manufacture RT-PCR test kits for Covid, followed by launching a home kit for Covid testing.
The TB kit will use the same technology – RT-PCR – as used in the Covid testing.
The ‘PathoDetect’ kit (MTB RIF and INH drug resistance) claims to detect TB accurately and completes automated testing of multiple samples within two hours.
“The move will be instrumental in supporting the honourable Prime Minister’s vision to eliminate TB by 2025 from India,” Gautam Wankhede, director of medical affairs at Mylab Discovery Solutions, told News18 in an exclusive interaction.
“Around 7 to 8 years ago, we zeroed down on tuberculosis as one of the top priority areas for the company and started working on this kit almost three to four years ago,” Wankhede said.
“But then Covid struck in 2020 and all resources were diverted to handle the pandemic and perhaps, the launch got delayed by one year.”
In May 2021, the ball started rolling again. In-house validations were done and the kit started getting clearances from the government bodies.
A team of 75 to 80 people were building the product with almost 20 people in the research and development wing and over 25 people working across India for field trials at nine centres across India. The remaining people worked in multiple other roles including technology and medical expertise.
How Does the Kit Work?
The kit allows testing of both pulmonary and non-pulmonary samples including sputum, cough, samples collected from lungs, and endometrial tissues.
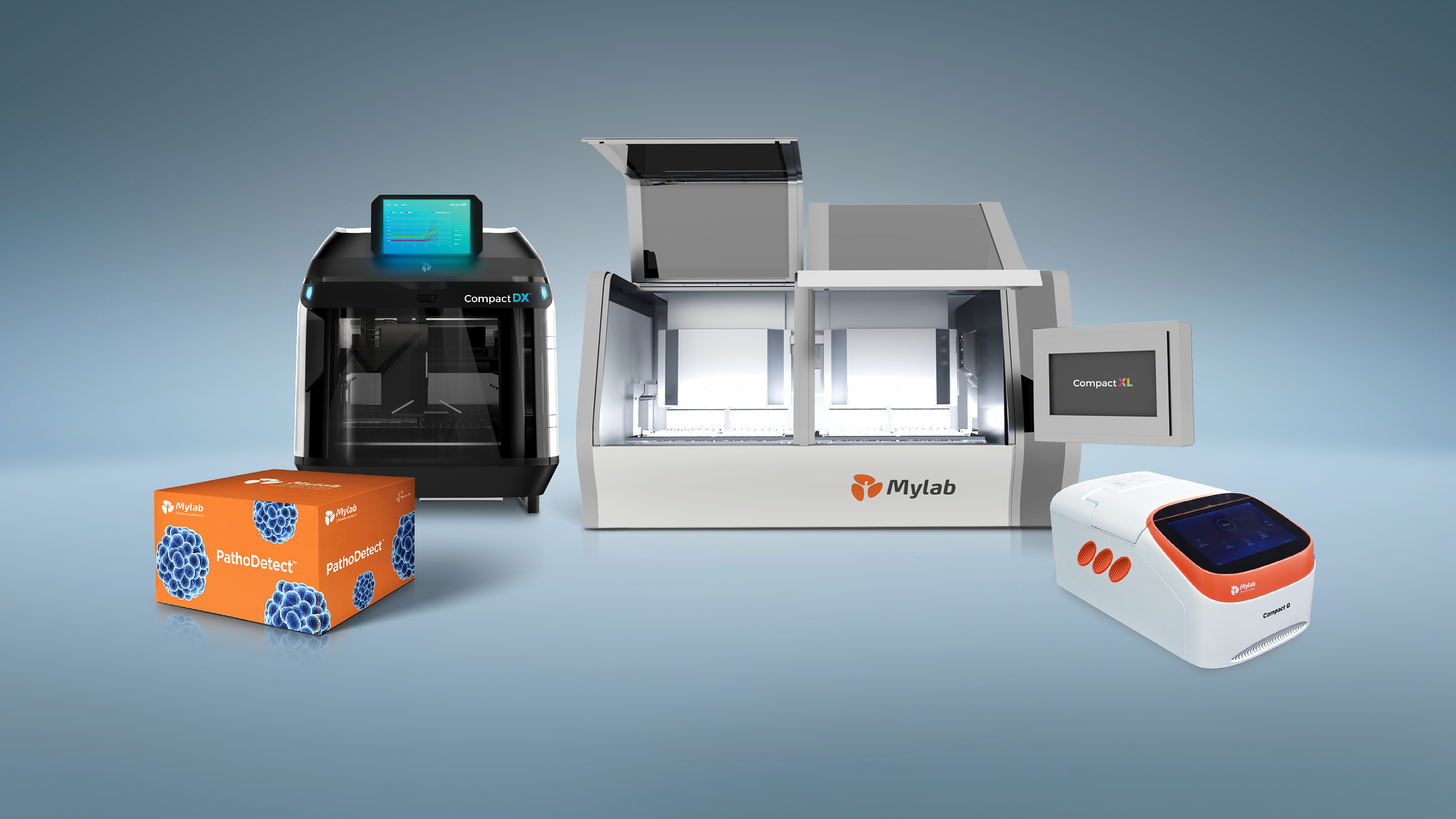
It is a molecular kit, which extracts the DNA information and identifies if the genes are of TB-causing bacteria.
According to Science Direct, RT PCR’s principle is to convert RNA into its complementary DNA sequence by reverse transcriptase, to synthesise a second strand with DNA polymerase, and finally to generate cDNA molecule, which can be amplified by PCR in the normal way.
Wankhede said one of the objectives behind making the kit was to speed up testing by automated systems that can do multiple tests at one time.
He pointed out that there is scarcity of trained manpower for RT-PCR testing, but, with this kit, the challenge can be overcome as it is a fully automated system, which does not need highly technical individuals to handle samples and reagents.
Also, he said the kit has been approved after rigorous and large-scale field trials and recommended by a TB expert committee under the aegis of ICMR.
Mylabs’ Kit vs Others
The centres of trials included the most reputed TB research centres of India, which evaluated the performance of the kit against the currently used diagnostic assays for tuberculosis, the company said.
It is by far the only kit, which conducts two tests in one go. With Mylab’s kit, patients can know their active TB infection as well as drug resistance to the two most common drugs — Isoniazid and Rifampacin — in a single test so that they take treatment that will actually work.
“With automation, the complete testing process can be conducted in rural areas and mobile labs. One has to just put the sample,” Wankhede said while adding that “the test kits have been designed to work in absolute room temperatures compared to existing PCR options, which need 2-8 Degree Celsius cold storage.”
Also, the kit allows scalable testing.
“While other machines allow one extraction at a time, this kit can be used in bigger centres, which collect 100 samples a day or even more as our machines work with 32 samples at a time with results in the next 2 hours,” he said.
The kit can be deployed in public hospitals and government-run primary healthcare centres and district-level hospitals as it is ICMR-approved. In the private market, the company will push the product via its sales team.
Currently, there are three ICMR-approved players in the TB detection kit market including Cepheid – an American molecular diagnostics company followed by Goa-based Molbio and now Mylabs.
“While Cepheid’s kits will not be cost-effective, Molbio kits do not test for resistance of two drugs,” he added.
While the company did not comment on the exact pricing and per-test cost, he said that “it will be much more cost-effective as compared to current options.”
The company plans to come up with other innovations in diagnostics in the field of public health concerns in the next six months.
Read all the Latest India News here




















Comments
0 comment